Nanosecond Plasmas in Liquids - How do they ignite ?
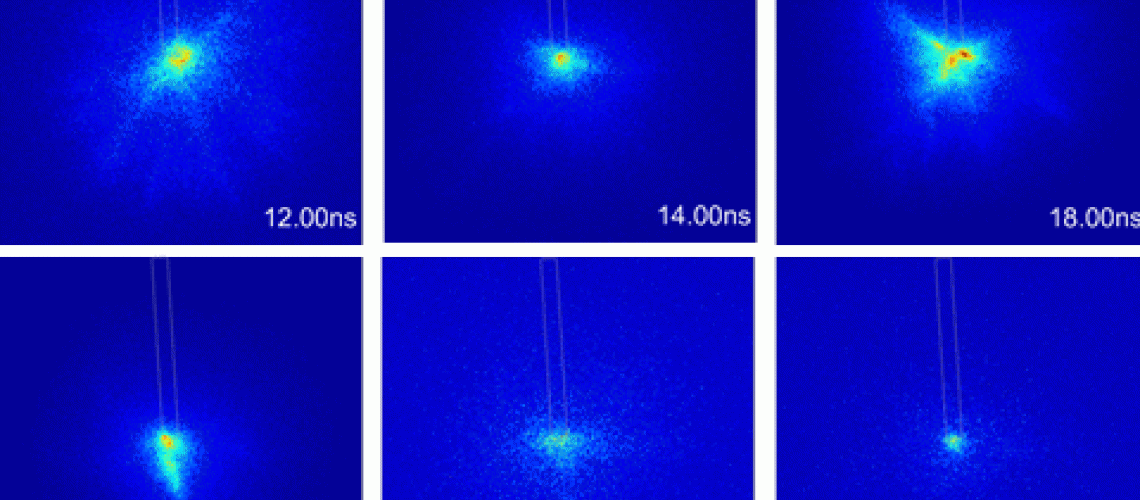
Discharges in liquids gained a huge interest over the last decades as they can be used for example for wastewater treatment, for nanoparticle formation as well as for biomedical applications . These types of discharges are appealing, because they produce a range of reactive species like OH and H2O2 inside water. The in-liquid discharges are usually ignited in a pin-to-pin or pin-to-plate configuration using a high voltage (HV) pulse applied to an electrode. The mechanism responsible for breakdown in liquids was initially associated with the breakdown of vapor in previously formed bubbles which are created at the tip of the powered electrode - such an the ignition environment is more gaseous than a direct liquid environment.
In comparison to that, nanosecond (ns) pulsed discharges with short rising times of the voltage of a few ns in water are assumed to ignite directly inside water . The ignition may occur via the Townsend mechanisms in the gas phase of vapor that is trapped in small nanovoids. Such nanovoids, may be created by the high electric fields that cause ruptures in the liquid. Alternatively, ignition may also occur by field emission and/or field ionization at the liquid solid interface. Due to the high electric field at the electrode tip, the potential barrier between the metal and adjacent water molecules is modified so that electrons are able to tunnel through the potential barrier. In case of a positive potential, this tunneling causes the formation of positive water ions by field ionization. In case of a negative voltage, tunneling causes the acceleration of electrons into the liquid, that may cause impact ionization of water molecules.
The understanding of the ignition process is crucial to understand the evolution of the chemistry of water dissociation. For example, if a bubble is present prior to ignition, a plasma from vapor is created and the dissociation products would then dissolve into the water at the end of the pulse. On the other hand, if the discharge is created directly inside water, aqueous electrons would be created and dissociation may occur directly in a very high pressure environment.
